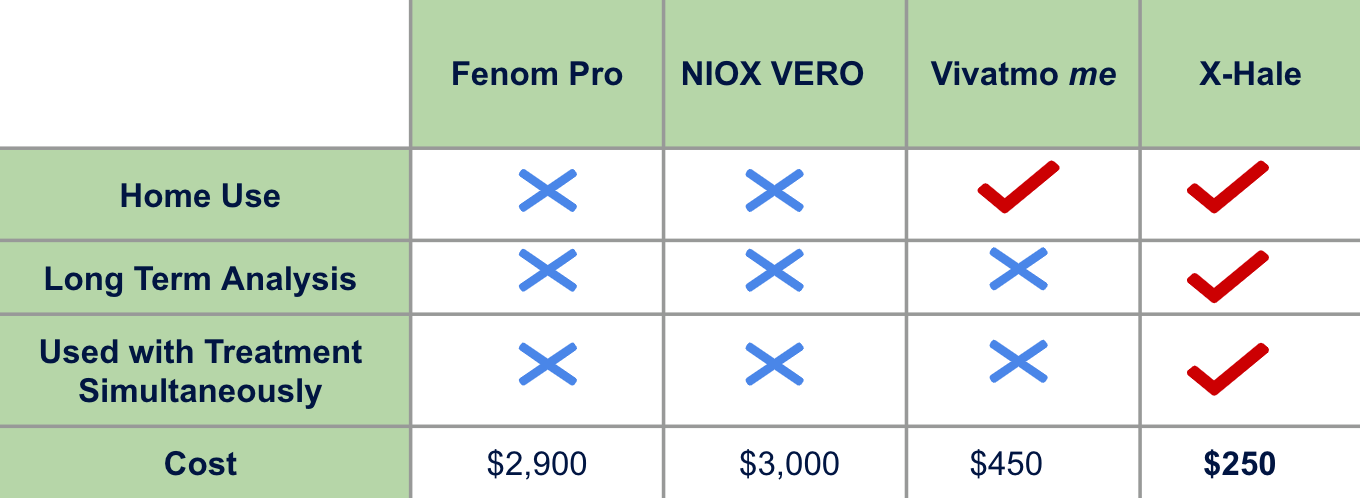
Market Strategy
From our company to your home
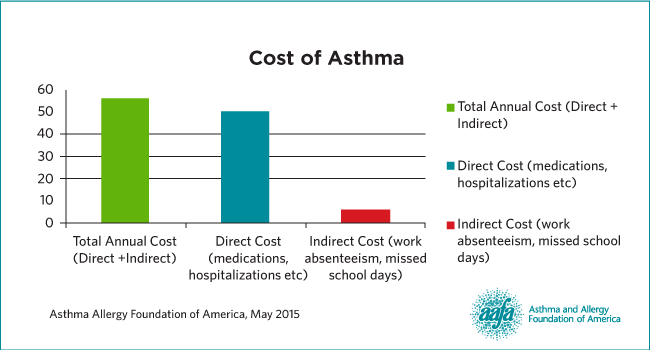
Cost of Asthma
On average, a person spends $3,266 on asthma-related costs. $1,830 was for prescriptions, $529 for hospitalizations, and $105 for emergency room care. Our company has high hopes to help patients save money by reducing the number of hospitalizations and improving asthma control (this includes the visits to the office, emergency room and outpatient).
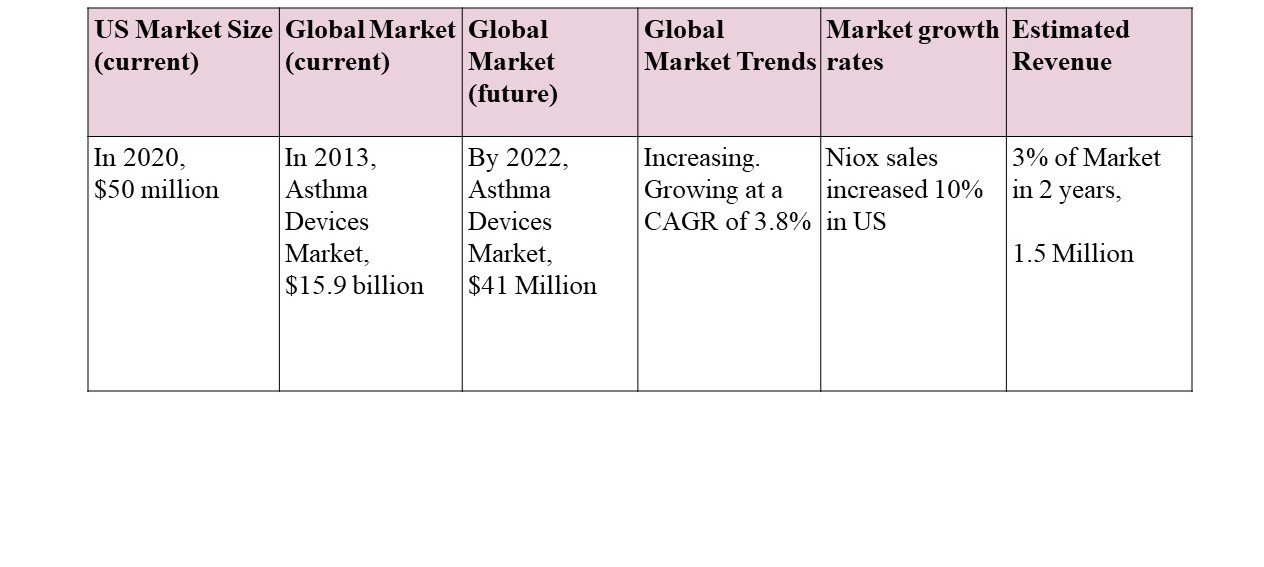
Asthma Market Growth
Marketing Plan
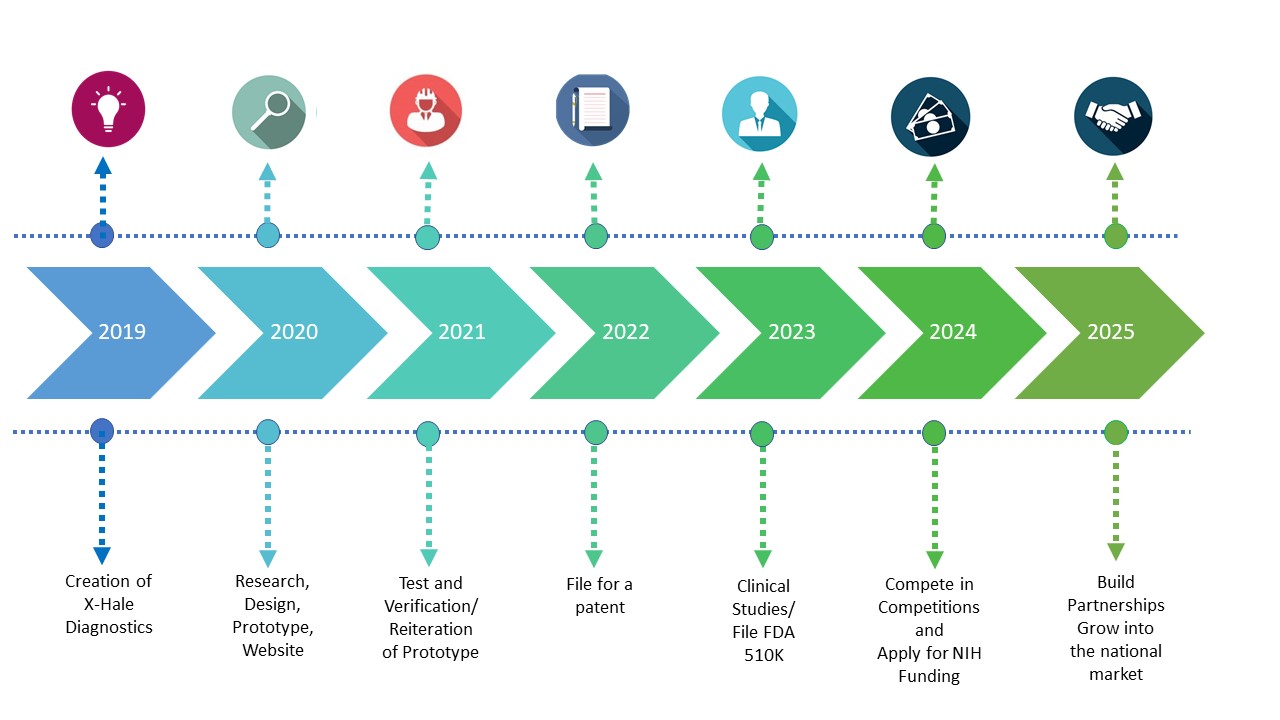
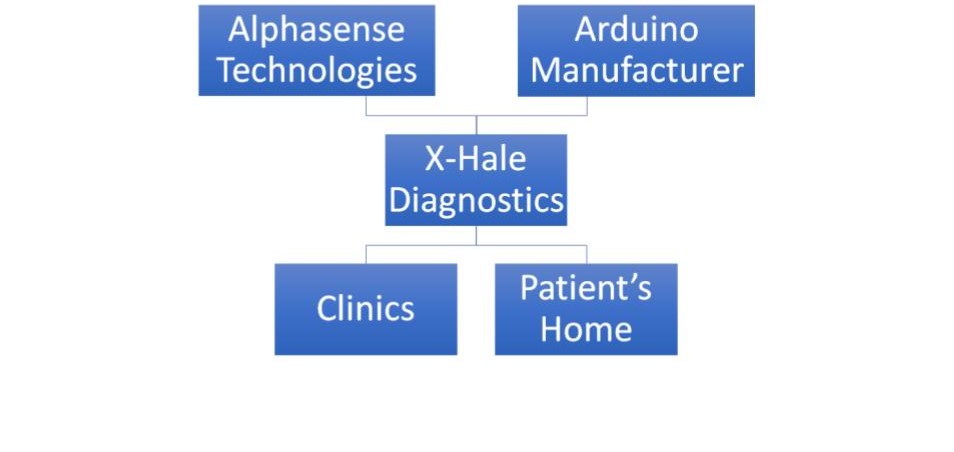
Phase 1:We will have a direct distribution channel by selling online. We will advertise by word-of-mouth, our website, and social media. We also plan to attend various medical device or asthma-related events to showcase our product. We will finish the prototype, and conduct testing and verification. Our key partners are our professors and our physician mentor. Our key supplier is Alphasense Technologies.
Phase 2:In this phase we will file for a patent, start clinical studies, and file for a 510K. We will participate in competitions and apply to the NIH for funding within the first five years. We will be expanding throughout California, and hiring more employees.
Phase 3:We will shift to an indirect distribution channel, and expand into the national market. Our goal is to have different intermediaries like a manufacturing department. We will also start partnerships or be bought by a larger company such as Propeller Health.